
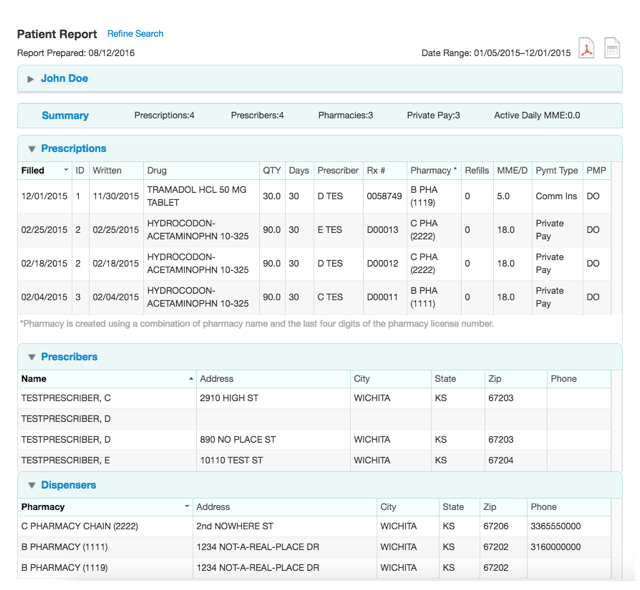
Information Available from the NJPMP
All New Jersey licensed pharmacies, including those located outside of New Jersey but registered as an out-of-State pharmacy with the New Jersey Board of Pharmacy, must transmit information about prescriptions the pharmacy fills for CDS (Schedule II-V) and HGH in an outpatient setting. This includes prescriptions that are shipped, mailed, distributed or delivered to a consumer in New Jersey.
All transactions for controlled dangerous substances (Schedule II-V) and HGH dispensed in, or into, New Jersey by pharmacists in an outpatient setting are reported to the PMP.
Data collection for the PMP began on September 1, 2011.
Data is submitted by the next business day from over 3,600 pharmacies (both in and out-of-state) on CDS (Schedule II-V) and HGH that have been dispensed.
Next
